Xylitol
Xylitol is a naturally occurring sugar alcohol derived from plants such as birch trees, corn cobs, and various fruits. Known for its hydrating, skin-barrier-strengthening, and antimicrobial properties, xylitol is used in skincare to boost moisture retention, improve skin resilience, and support overall skin health.
One of xylitol’s standout benefits is its humectant ability, meaning it helps attract and retain moisture in the skin, similar to glycerin or hyaluronic acid. By preventing transepidermal water loss (TEWL), xylitol ensures the skin remains hydrated, plump, and smooth. This makes it particularly beneficial for individuals with dry or dehydrated skin.
Beyond hydration, xylitol has been shown to strengthen the skin barrier by increasing ceramide production, which helps the skin defend itself against external irritants, pollutants, and bacteria. This makes it an effective ingredient for sensitive or compromised skin, as it supports long-term skin health and resilience.
Xylitol also possesses antimicrobial properties, which can help reduce the growth of acne-causing bacteria and maintain a balanced skin microbiome. This makes it useful in formulations for acne-prone and oily skin, where it helps regulate moisture levels without clogging pores. Additionally, xylitol has been studied for its ability to reduce inflammation and redness, making it beneficial for those with rosacea, eczema, or sensitive skin conditions.
Commonly found in hydrating serums, moisturizers, cleansers, and even oral care products, xylitol pairs well with other moisture-boosting ingredients like hyaluronic acid, glycerin, and ceramides. Its ability to lock in hydration, protect the skin barrier, and provide antimicrobial support makes it a versatile and valuable ingredient in modern skincare formulations.
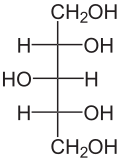
Xylitol is a chemical compound with the formula C
5H
12O
5, or HO(CH2)(CHOH)3(CH2)OH; specifically, one particular stereoisomer with that structural formula. It is a colorless or white crystalline solid. It is classified as a polyalcohol and a sugar alcohol, specifically an alditol. Of the common sugar alcohols, only sorbitol is more soluble in water.
 | |
 Xylitol crystals
| |
| Names | |
|---|---|
| Pronunciation | /ˈzaɪlɪtɒl/ |
| IUPAC name
meso-Xylitol
| |
| Systematic IUPAC name
(2R,3r,4S)-Pentane-1,2,3,4,5-pentol | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.626 |
| E number | E967 (glazing agents, ...) |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H12O5 | |
| Molar mass | 152.146 g·mol−1 |
| Density | 1.52 g/cm3 |
| Melting point | 92 to 96 °C (198 to 205 °F; 365 to 369 K) |
| Boiling point | 345.39 °C (653.70 °F; 618.54 K) Predicted value using Adapted Stein & Brown method |
| 168 g/100 g | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Related alkanes
|
Pentane |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
The name derives from Ancient Greek: ξύλον, xyl[on] 'wood', with the suffix -itol used to denote it being a sugar alcohol.
Xylitol is used as a food additive and sugar substitute. Its European Union code number is E967. Replacing sugar with xylitol in food products may promote better dental health, but evidence is lacking on whether xylitol itself prevents dental cavities. In the United States, xylitol is used as a common sugar substitute, and is considered to be safe for humans.
Xylitol can be toxic to dogs.

