Tricaprylin
Tricaprylin is a widely used ingredient in cosmetics, personal care, and skincare formulations. Depending on its function, it may serve as a moisturizer, preservative, emulsifier, or active ingredient to enhance the overall effectiveness and performance of a product.
This article may be unbalanced toward certain viewpoints. (April 2015) |
Axona was previously marketed as a medical food for the clinical dietary management of the impairment of metabolic processes associated with mild to moderate Alzheimer's disease. It is a proprietary formulation of fractionated palm kernel oil (caprylic triglyceride), a medium-chain triglyceride. Cericin, the company that makes Axona, states that during digestion, caprylic triglyceride is broken down into ketones, which provide an alternative energy source for the brain. Its use is based on the idea that the brain's ability to use its normal energy source, glucose, is impaired in Alzheimer's disease. Axona was first sold in March 2009.
 | |
 | |
| Names | |
|---|---|
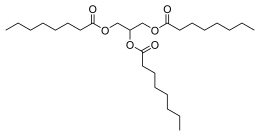
| IUPAC name
Tri-O-octanoylglycerol
| |
| Systematic IUPAC name
Propane-1,2,3-triyl tri(octanoate) | |
| Other names
Glycerol trioctanoate; Tricaprylin; [2-Octanoyloxy-1-(octanoyloxymethyl)ethyl] octanoate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.007.898 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C27H50O6 | |
| Molar mass | 470.691 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
In 2013, US Food and Drug Administration (FDA) determined Axona was misbranded because the product was labeled and marketed as a medical food but does not meet the statutory definition of a medical food. Axona has not been approved by the FDA as a drug to treat Alzheimer's and the efficacy of managing the health of Alzheimer's patients by use of this medical food has been questioned by experts in the field, including the Alzheimer's Association.

