Squalene
Squalene is a widely used ingredient in cosmetics, personal care, and skincare formulations. Depending on its function, it may serve as a moisturizer, preservative, emulsifier, or active ingredient to enhance the overall effectiveness and performance of a product.
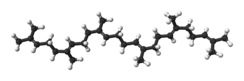
Squalene is an organic compound. It is a triterpene with the formula C30H50. It is a colourless oil, although impure samples appear yellow. It was originally obtained from shark liver oil (hence its name, as Squalus is a genus of sharks). An estimated 12% of bodily squalene in humans is found in sebum. Squalene has a role in topical skin lubrication and protection.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(6E,10E,14E,18E)-2,6,10,15,19,23-Hexamethyltetracosa-2,6,10,14,18,22-hexaene | |
| Identifiers | |
3D model (JSmol)
|
|
| 1728919 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.479 |
| EC Number |
|
| KEGG | |
| MeSH | Squalene |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C30H50 | |
| Molar mass | 410.730 g·mol−1 |
| Appearance | Colourless oil |
| Density | 0.858 g·cm−3 |
| Melting point | −5 °C (23 °F; 268 K) |
| Boiling point | 285 °C (545 °F; 558 K) at 3.3 kPa |
| log P | 12.188 |
Refractive index (nD)
|
1.4956 (at 20 °C) |
| Viscosity | 12 cP (at 20 °C) |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 110 °C (230 °F; 383 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Most plants, fungi, and animals produce squalene as biochemical precursor in sterol biosynthesis, including cholesterol and steroid hormones in the human body. It is also an intermediate in the biosynthesis of hopanoids in many bacteria.
Squalene is an important ingredient in some vaccine adjuvants: The Novartis and GlaxoSmithKline adjuvants are called MF59 and AS03, respectively.


