Sodium Saccharin
Sodium Saccharin is a widely used ingredient in cosmetics, personal care, and skincare formulations. Depending on its function, it may serve as a moisturizer, preservative, emulsifier, or active ingredient to enhance the overall effectiveness and performance of a product.
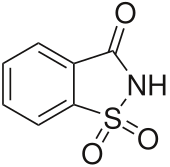
Saccharin, also called saccharine, benzosulfimide, or E954, or used in saccharin sodium or saccharin calcium forms, is a non-nutritive artificial sweetener. Saccharin is a sultam that is about 500 times sweeter than sucrose, but has a bitter or metallic aftertaste, especially at high concentrations. It is used to sweeten products, such as drinks, candies, baked goods, tobacco products, excipients, and for masking the bitter taste of some medicines. It appears as white crystals and is odorless.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1H-1λ6,2-Benzothiazole-1,1,3(2H)-trione | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.202 |
| E number | E954 (glazing agents, ...) |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C7H5NO3S | |
| Molar mass | 183.18 g·mol−1 |
| Appearance | White crystalline solid |
| Density | 0.828 g/cm3 |
| Melting point | 228.8 to 229.7 °C (443.8 to 445.5 °F; 501.9 to 502.8 K) |
| 1 g per 290 mL | |
| Acidity (pKa) | 1.6 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |

