Sodium benzoate
« Back to Glossary Index
« Back to Glossary Index
Sodium benzoate is a widely used ingredient in cosmetics, personal care, and skincare formulations. Depending on its function, it may serve as a moisturizer, preservative, emulsifier, or active ingredient to enhance the overall effectiveness and performance of a product.
Sodium benzoate (Wikipedia)
Sodium benzoate also known as benzoate of soda is the sodium salt of benzoic acid, widely used as a food preservative (with an E number of E211) and a pickling agent. It appears as a white crystalline chemical with the formula C6H5COONa.
 | |
 | |
 Ball-and-stick model of part of the crystal structure
| |
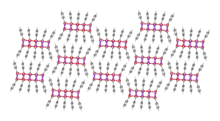 Ball-and-stick model of packing in the crystal structure
| |
| Names | |
|---|---|
| Preferred IUPAC name
Sodium benzoate | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.760 |
| E number | E211 (preservatives) |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C7H5NaO2 | |
| Molar mass | 144.105 g·mol−1 |
| Appearance | white or colourless crystalline powder |
| Odor | odorless |
| Density | 1.497 g/cm3 |
| Melting point | 410 °C (770 °F; 683 K) |
| 62.65 g/100 mL (0 °C) 62.84 g/100 mL (15 °C) 62.87 g/100 mL (30 °C) 74.2 g/100 mL (100 °C) | |
| Solubility | soluble in liquid ammonia, pyridine |
| Solubility in methanol | 8.22 g/100 g (15 °C) 7.55 g/100 g (66.2 °C) |
| Solubility in ethanol | 2.3 g/100 g (25 °C) 8.3 g/100 g (78 °C) |
| Solubility in 1,4-Dioxane | 0.818 mg/kg (25 °C) |
| Pharmacology | |
| A16AX11 (WHO) | |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H319 | |
| P305+P351+P338 | |
| NFPA 704 (fire diamond) | |
| Flash point | 100 °C (212 °F; 373 K) |
| 500 °C (932 °F; 773 K) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
4100 mg/kg (oral, rat) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |


