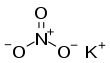Potassium nitrate
Potassium nitrate is a widely used ingredient in cosmetics, personal care, and skincare formulations. Depending on its function, it may serve as a moisturizer, preservative, emulsifier, or active ingredient to enhance the overall effectiveness and performance of a product.
Potassium nitrate is a chemical compound with a sharp, salty, bitter taste and the chemical formula KNO3. It is a potassium salt of nitric acid. This salt consists of potassium cations K+ and nitrate anions NO−3, and is therefore an alkali metal nitrate. It occurs in nature as a mineral, niter (or nitre outside the US). It is a source of nitrogen, and nitrogen was named after niter. Potassium nitrate is one of several nitrogen-containing compounds collectively referred to as saltpetre (or saltpeter in the US).
 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Potassium nitrate
| |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.028.926 | ||
| EC Number |
| ||
| E number | E252 (preservatives) | ||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1486 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| KNO3 | |||
| Molar mass | 101.1032 g/mol | ||
| Appearance | white solid | ||
| Odor | odorless | ||
| Density | 2.109 g/cm3 (16 °C) | ||
| Melting point | 334 °C (633 °F; 607 K) | ||
| Boiling point | 400 °C (752 °F; 673 K) (decomposes) | ||
| |||
| Solubility | slightly soluble in ethanol soluble in glycerol, ammonia | ||
| Basicity (pKb) | 15.3 | ||
| −33.7·10−6 cm3/mol | |||
Refractive index (nD)
|
1.335, 1.5056, 1.5604 | ||
| Structure | |||
| Orthorhombic, Aragonite | |||
| Thermochemistry | |||
Heat capacity (C)
|
95.06 J/mol K | ||
Std enthalpy of
formation (ΔfH⦵298) |
−494.00 kJ/mol | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Oxidant, harmful if swallowed, inhaled, or absorbed on skin. Causes irritation to skin and eye area. | ||
| GHS labelling: | |||
 
| |||
| H272, H315, H319, H335 | |||
| P102, P210, P220, P221, P280 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | non-flammable (oxidizer) | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
1901 mg/kg (oral, rabbit) 3750 mg/kg (oral, rat) | ||
| Safety data sheet (SDS) | ICSC 0184 | ||
| Related compounds | |||
Other anions
|
Potassium nitrite | ||
Other cations
|
|||
Related compounds
|
|||
| Supplementary data page | |||
| Potassium nitrate (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Major uses of potassium nitrate are in fertilizers, tree stump removal, rocket propellants and fireworks. It is one of the major constituents of traditional gunpowder (black powder). In processed meats, potassium nitrate reacts with hemoglobin and myoglobin generating a red color, becoming highly toxic and carcinogenic.