Perfluorohexane
Perfluorohexane is a widely used ingredient in cosmetics, personal care, and skincare formulations. Depending on its function, it may serve as a moisturizer, preservative, emulsifier, or active ingredient to enhance the overall effectiveness and performance of a product.
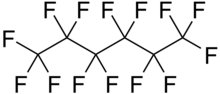
Perfluorohexane (C6F14), or tetradecafluorohexane, is a fluorocarbon. It is a derivative of hexane in which all the hydrogen atoms are replaced by fluorine atoms. It is used in one formulation of the electronic cooling liquid/insulator Fluorinert for low-temperature applications due to its low boiling point of 56 °C and freezing point of −90 °C. It is odorless and colorless. Unlike typical hydrocarbons, the structure features a helical carbon backbone. In medical imaging it is used as a contrast agent.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Tetradecafluorohexane | |
| Other names
FC-72,
Fluorinert FC-72, Flutec PP1, Perfluoro-compound FC-72 | |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | PFH |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.005.987 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6F14 | |
| Molar mass | 338.041845 |
| Appearance | Clear, colorless |
| Odor | Odorless |
| Density | 1.680 kg/m3 (Liquid) |
| Melting point | −90 °C (−130 °F; 183 K) |
| Boiling point | 56 °C (133 °F; 329 K) |
| Vapor pressure | 30.9 kPa (25 °C) |
| Thermal conductivity | 0.057 W/(m·K) |
| Viscosity | 0.64 cP |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
> 5 g/kg (rat, oral) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |


