Isopentane
Isopentane is a widely used ingredient in cosmetics, personal care, and skincare formulations. Depending on its function, it may serve as a moisturizer, preservative, emulsifier, or active ingredient to enhance the overall effectiveness and performance of a product.
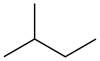
Isopentane, also called methylbutane or 2-methylbutane, is a branched-chain saturated hydrocarbon (an alkane) with five carbon atoms, with formula C
5H
12 or CH(CH
3)
2(C
2H
5).
 | |
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methylbutane | |
| Other names
Isopentane
| |
| Identifiers | |
3D model (JSmol)
|
|
| 1730723 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.001.039 |
| EC Number |
|
| 49318 | |
| MeSH | isopentane |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1265 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H12 | |
| Molar mass | 72.151 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Gasoline-like |
| Density | 616 mg mL−1 |
| Melting point | −161 to −159 °C; −258 to −254 °F; 112 to 114 K |
| Boiling point | 27.8 to 28.2 °C; 81.9 to 82.7 °F; 300.9 to 301.3 K |
| Vapor pressure | 76.992 kPa (at 20 °C) |
Henry's law
constant (kH) |
7.2 nmol Pa−1 kg−1 |
| UV-vis (λmax) | 192 nm |
Refractive index (nD)
|
1.354 |
| Viscosity | 0.214 cP (at 20 °C) |
| Thermochemistry | |
Heat capacity (C)
|
164.85 J K−1 mol−1 |
Std molar
entropy (S⦵298) |
260.41 J K−1 mol−1 |
Std enthalpy of
formation (ΔfH⦵298) |
−179.1–−177.3 kJ mol−1 |
Std enthalpy of
combustion (ΔcH⦵298) |
~ 3.3 MJ mol−1, 19,664 Btu/lb |
| Hazards | |
| GHS labelling: | |
   
| |
| Danger | |
| H224, H301, H302, H305, H336, H411 | |
| P210, P261, P273, P301+P310, P331 | |
| NFPA 704 (fire diamond) | |
| Flash point | −51 °C (−60 °F; 222 K) |
| 420 °C (788 °F; 693 K) | |
| Explosive limits | 1.4–8.3% |
| Related compounds | |
Related alkanes
|
|
Related compounds
|
2-Ethyl-1-butanol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Isopentane is a volatile and flammable liquid. It is one of three structural isomers with the molecular formula C5H12, the others being pentane (n-pentane) and neopentane (2,2-dimethylpropane).
Isopentane is commonly used in conjunction with liquid nitrogen to achieve a liquid bath temperature of −160 °C. Natural gas typically contains 1% or less isopentane, but it is a significant component of natural gasoline.


