Hydrogenated oils
Hydrogenated oils are oils that have been chemically altered through a process called hydrogenation, which adds hydrogen to their molecular structure, transforming them from liquid to semi-solid or solid form. This modification increases their stability, prevents oxidation, and extends their shelf life, making them a popular ingredient in skincare products. Hydrogenated oils function primarily as emollients, helping to soften and smooth the skin by creating a protective barrier that locks in moisture. This makes them especially beneficial in formulations designed for dry or sensitive skin, as they provide long-lasting hydration and prevent transepidermal water loss (TEWL).
In skincare, hydrogenated oils are often found in creams, lotions, lip balms, and body butters, where they contribute to a rich, luxurious texture. Commonly used examples include hydrogenated vegetable oil, hydrogenated castor oil, and hydrogenated soybean oil, all of which help enhance the spreadability and stability of formulations. Unlike their partially hydrogenated counterparts found in food, which can produce harmful trans fats, fully hydrogenated oils in skincare do not pose the same health concerns. Instead, they act as conditioning agents that help improve skin suppleness and protect against environmental stressors.
While hydrogenated oils are effective at providing moisture and enhancing product consistency, their occlusive nature may not be suitable for all skin types. People with acne-prone or oily skin may find that these ingredients contribute to clogged pores, depending on the formulation. Additionally, some consumers prefer to avoid hydrogenated oils in favor of naturally derived plant butters and oils that offer similar hydration benefits without chemical modification. However, in well-formulated products, hydrogenated oils can play a valuable role in maintaining skin barrier function, reducing dryness, and enhancing the sensory experience of skincare applications.
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces double and triple bonds in hydrocarbons.
| Hydrogenation | |
|---|---|
| Conditions | |
| Catalyst | Ni, Pd, Pt |
| Process type | Chemical |
|---|---|
| Industrial sector(s) | Food industry, petrochemical industry, pharmaceutical industry, agricultural industry |
| Main technologies or sub-processes | Various transition metal catalysts, high-pressure technology |
| Feedstock | Unsaturated substrates and hydrogen or hydrogen donors |
| Product(s) | Saturated hydrocarbons and derivatives |
| Inventor | Paul Sabatier |
| Year of invention | 1897 |
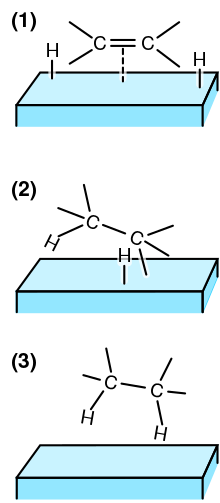
(1) The reactants are adsorbed on the catalyst surface and H2 dissociates.
(2) An H atom bonds to one C atom. The other C atom is still attached to the surface.
(3) A second C atom bonds to an H atom. The molecule leaves the surface.

