Citric acid
Citric acid is a widely used ingredient in cosmetics, personal care, and skincare formulations. Depending on its function, it may serve as a moisturizer, preservative, emulsifier, or active ingredient to enhance the overall effectiveness and performance of a product.
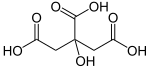
Citric acid is an organic compound with the formula C6H8O7. It is a colorless weak organic acid. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the metabolism of all aerobic organisms.
| |||
 | |||
| Names | |||
|---|---|---|---|
| IUPAC name
Citric acid
| |||
| Preferred IUPAC name
2-Hydroxypropane-1,2,3-tricarboxylic acid | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.973 | ||
| EC Number |
| ||
| E number | E330 (antioxidants, ...) | ||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C6H8O7 | |||
| Molar mass | 192.123 g/mol (anhydrous), 210.14 g/mol (monohydrate) | ||
| Appearance | white solid | ||
| Odor | Odorless | ||
| Density | 1.665 g/cm3 (anhydrous) 1.542 g/cm3 (18 °C, monohydrate) | ||
| Melting point | 156 °C (313 °F; 429 K) | ||
| Boiling point | 310 °C (590 °F; 583 K) decomposes from 175 °C | ||
| 54% w/w (10 °C) 59.2% w/w (20 °C) 64.3% w/w (30 °C) 68.6% w/w (40 °C) 70.9% w/w (50 °C) 73.5% w/w (60 °C) 76.2% w/w (70 °C) 78.8% w/w (80 °C) 81.4% w/w (90 °C) 84% w/w (100 °C) | |||
| Solubility | Soluble in acetone, alcohol, ether, ethyl acetate, DMSO Insoluble in C 6H 6, CHCl3, CS2, toluene | ||
| Solubility in ethanol | 62 g/100 g (25 °C) | ||
| Solubility in amyl acetate | 4.41 g/100 g (25 °C) | ||
| Solubility in diethyl ether | 1.05 g/100 g (25 °C) | ||
| Solubility in 1,4-dioxane | 35.9 g/100 g (25 °C) | ||
| log P | −1.64 | ||
| Acidity (pKa) | pKa1 = 3.13 pKa2 = 4.76 pKa3 = 6.39, 6.40 pKa4 = 14.4 | ||
Refractive index (nD)
|
1.493–1.509 (20 °C) 1.46 (150 °C) | ||
| Viscosity | 6.5 cP (50% aq. sol.) | ||
| Structure | |||
| Monoclinic | |||
| Thermochemistry | |||
Heat capacity (C)
|
226.51 J/(mol·K) (26.85 °C) | ||
Std molar
entropy (S⦵298) |
252.1 J/(mol·K) | ||
Std enthalpy of
formation (ΔfH⦵298) |
−1543.8 kJ/mol | ||
| 1985.3 kJ/mol (474.5 kcal/mol, 2.47 kcal/g), 1960.6 kJ/mol 1972.34 kJ/mol (471.4 kcal/mol, 2.24 kcal/g) (monohydrate) | |||
| Pharmacology | |||
| A09AB04 (WHO) | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Skin and eye irritant | ||
| GHS labelling: | |||
 
| |||
| Warning | |||
| H290, H319, H315 | |||
| P305+P351+P338 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 155 °C (311 °F; 428 K) | ||
| 345 °C (653 °F; 618 K) | |||
| Explosive limits | 8% | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
3000 mg/kg (rats, oral) | ||
| Safety data sheet (SDS) | HMDB (PDF) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
More than two million tons of citric acid are manufactured every year. It is used widely as acidifier, flavoring, preservative, and chelating agent.
A citrate is a derivative of citric acid; that is, the salts, esters, and the polyatomic anion found in solutions and salts of citric acid. An example of the former, a salt is trisodium citrate; an ester is triethyl citrate. When citrate trianion is part of a salt, the formula of the citrate trianion is written as C
6H
5O3−
7 or C
3H
5O(COO)3−
3.