Bronopol
Bronopol is a widely used ingredient in cosmetics, personal care, and skincare formulations. Depending on its function, it may serve as a moisturizer, preservative, emulsifier, or active ingredient to enhance the overall effectiveness and performance of a product.
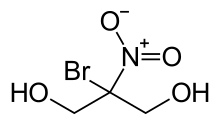
Bronopol (INN; chemical name 2-bromo-2-nitropropane-1,3-diol) is an organic compound that is used as an antimicrobial. It is a white solid although commercial samples appear yellow.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-Bromo-2-nitropropane-1,3-diol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.131 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 3241 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H6BrNO4 | |
| Molar mass | 199.988 g·mol−1 |
| Appearance | White solid |
| Density | 1.1 g/cm3 |
| Melting point | 130 °C (266 °F; 403 K) |
| Boiling point | 140 °C (284 °F; 413 K) (decomposes) |
| Pharmacology | |
| QD01AE91 (WHO) | |
| Hazards | |
| GHS labelling: | |
  
| |
| Danger | |
| H302, H312, H315, H318, H335, H400 | |
| P261, P264, P270, P271, P273, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P310, P312, P321, P322, P330, P332+P313, P362, P363, P391, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
The first reported synthesis of bronopol was in 1897.[citation needed]
Bromopol was invented by The Boots Company PLC in the early 1960s and first applications were as a preservative for pharmaceuticals. Due to its low mammalian toxicity at in-use levels and high activity against bacteria, especially Gram-negative species, bronopol became popular as a preservative in many consumer products such as shampoos and cosmetics. It was subsequently adopted as an antimicrobial in other industrial environments such as paper mills, oil exploration, and production facilities, as well as cooling water disinfection plants.

